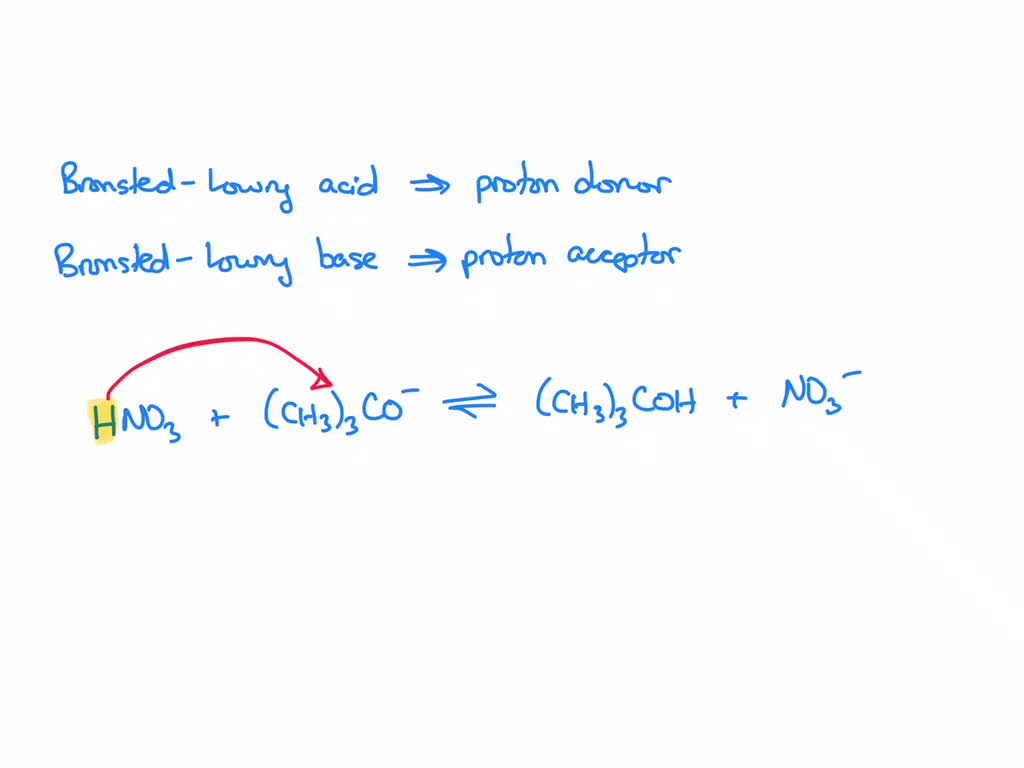
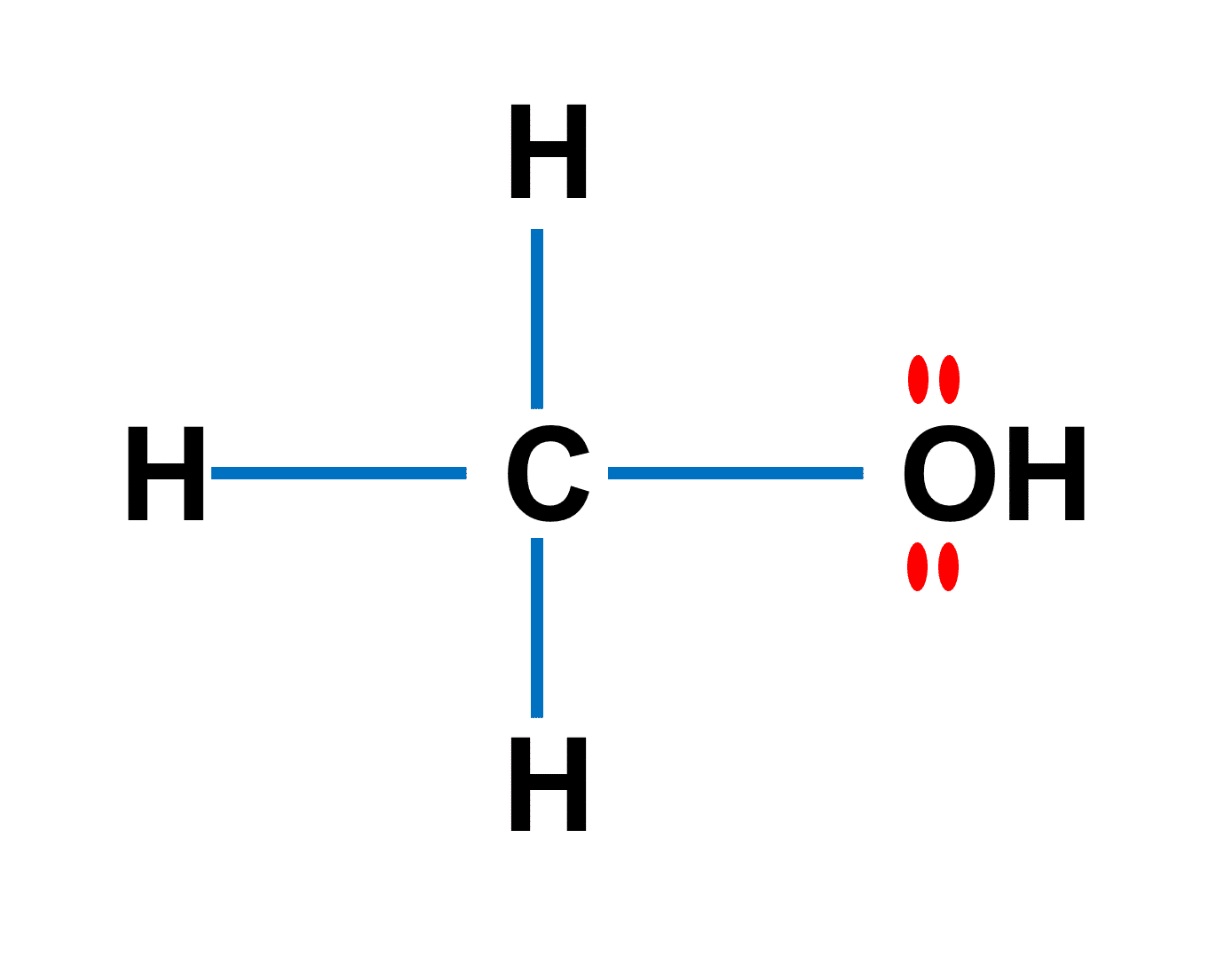
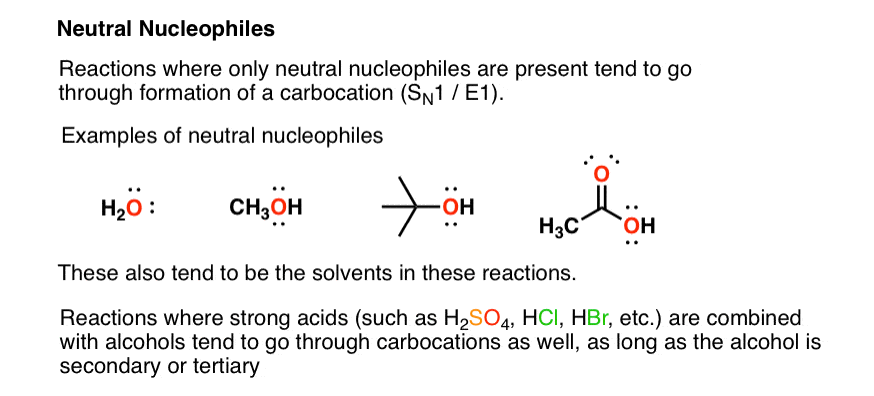
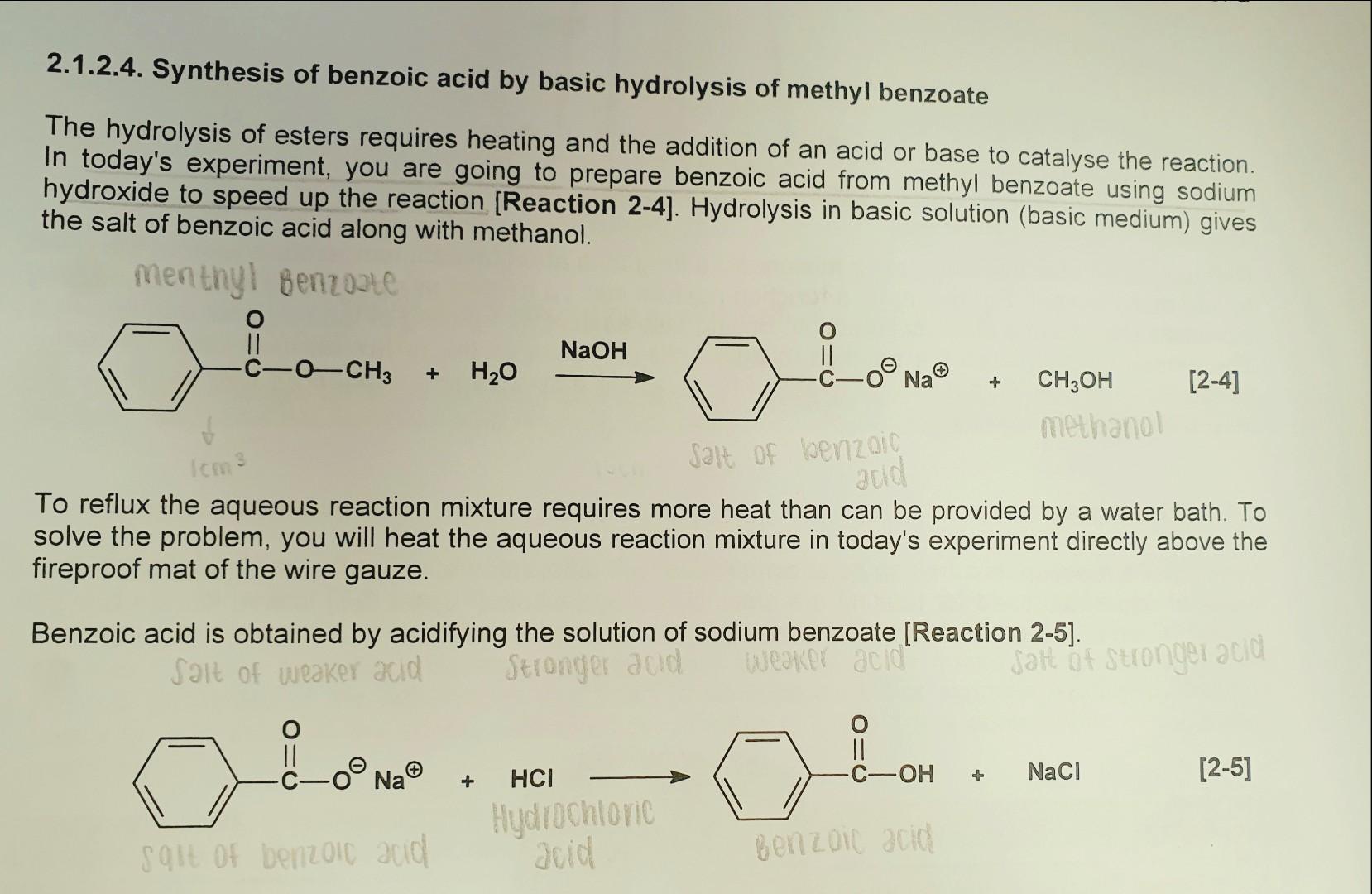
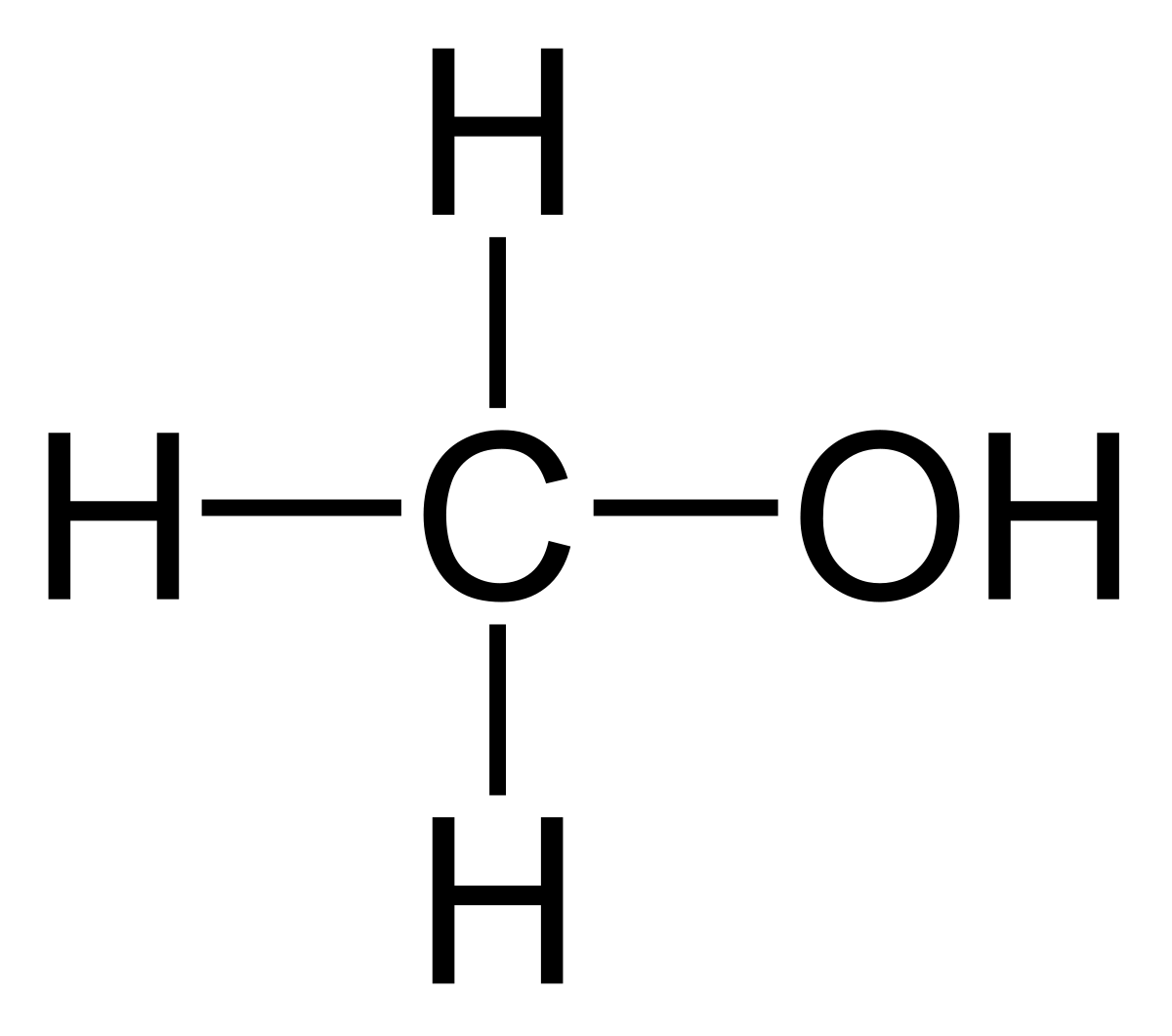
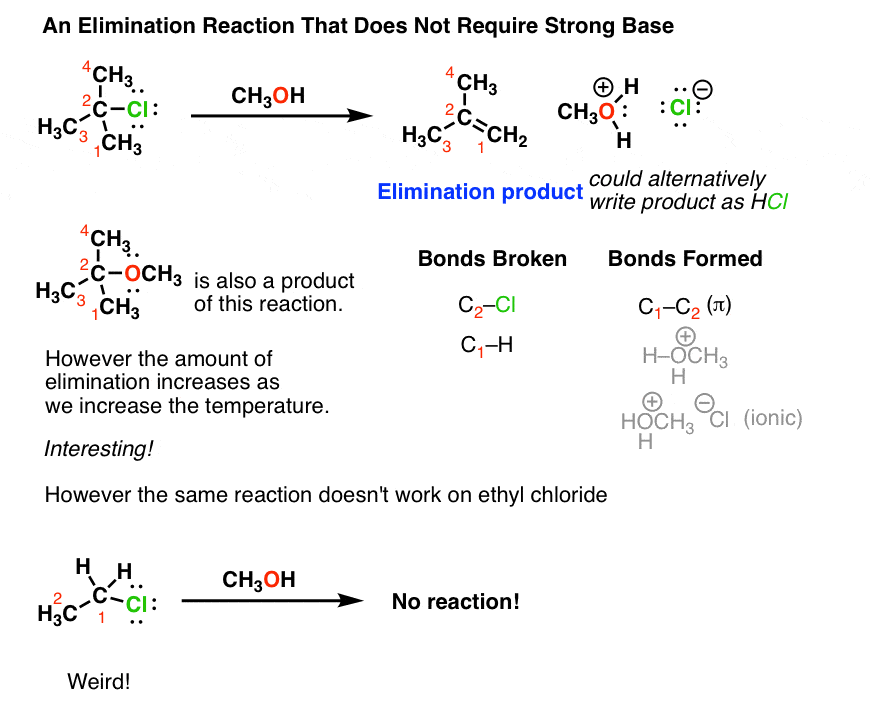
Can methanol be classified as an Arrhenius acid? To my knowledge it does not want the H from the O--H bond to dissociate : r/Mcat

Catalysts | Free Full-Text | Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors

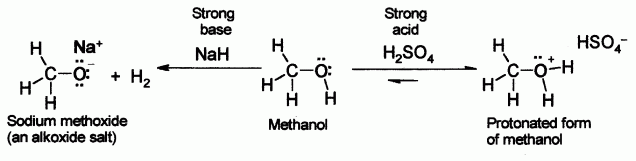
SOLVED:Alcohols can act either as weak acids or as weak bases, just as water can. Show the reaction of methanol, CH3 OH, with a strong acid such as HCl and with a
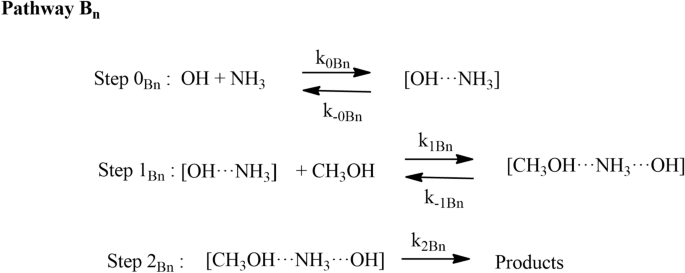
Effect of ammonia and water molecule on OH + CH3OH reaction under tropospheric condition | Scientific Reports
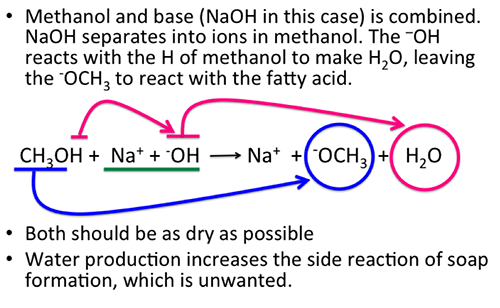
9.2 The Reaction of Biodiesel: Transesterification | EGEE 439: Alternative Fuels from Biomass Sources

Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2 | Nature Catalysis
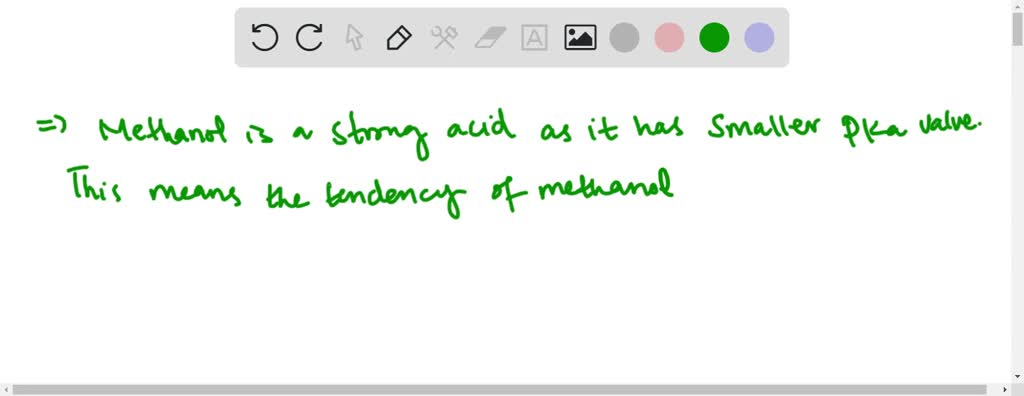
SOLVED:Does methanol behave as an acid or a base when it reacts with methylamine? (Hint: See page 55 for the structures of methanol and methylamine.)