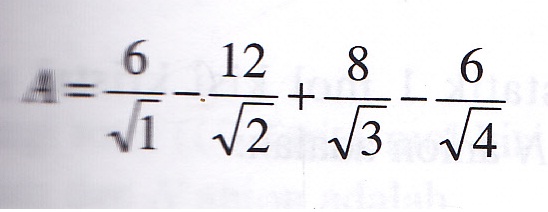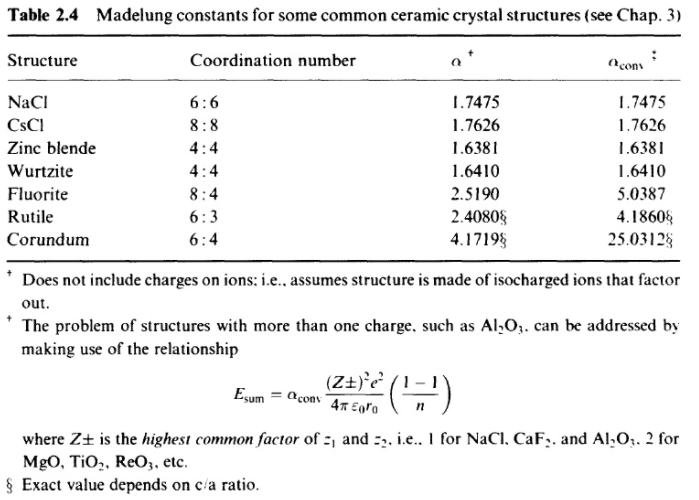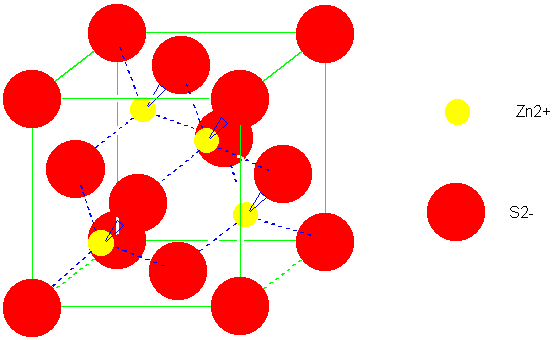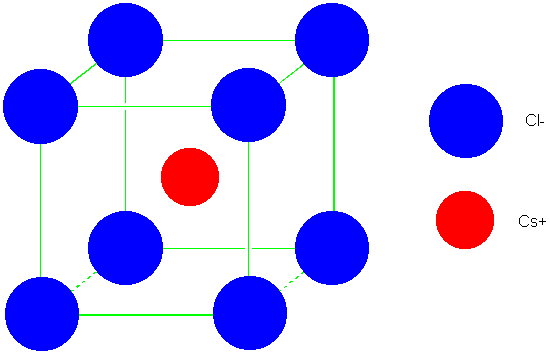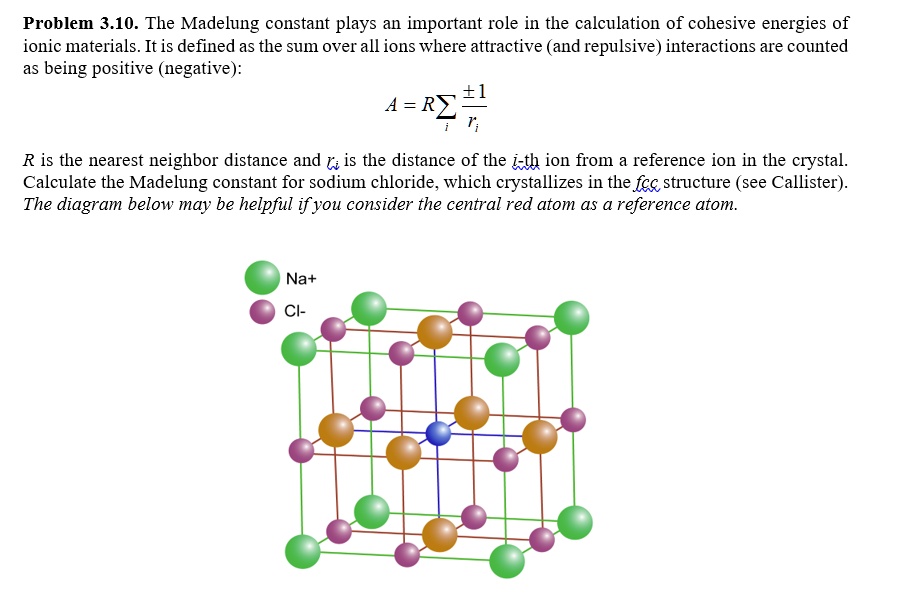
SOLVED: Problem 3.10. The Madelung constant plays an important role in the calculation of cohesive energies of ionic materials. It is defined as the sum over all ions where attractive (and repulsive)

Write the first five terms of the Madelung constant for a two-dimensional lattice of alternating positive and negative ions. | Homework.Study.com
Show that the Madelung constant for a one-dimensional array of ions of alternating sign with - Sarthaks eConnect | Largest Online Education Community

Calculate the lattice energy of sodium chloride crystal from the following data: Born exponent, n=8, Madelung constant for NaCl=1.748, Ionic radius of Na^(+)=0.95 Å, Ionic radius of Cl^(-)=1.81Å.

solid state physics - Madelung constant for a two dimensional square crystal lattice - Physics Stack Exchange