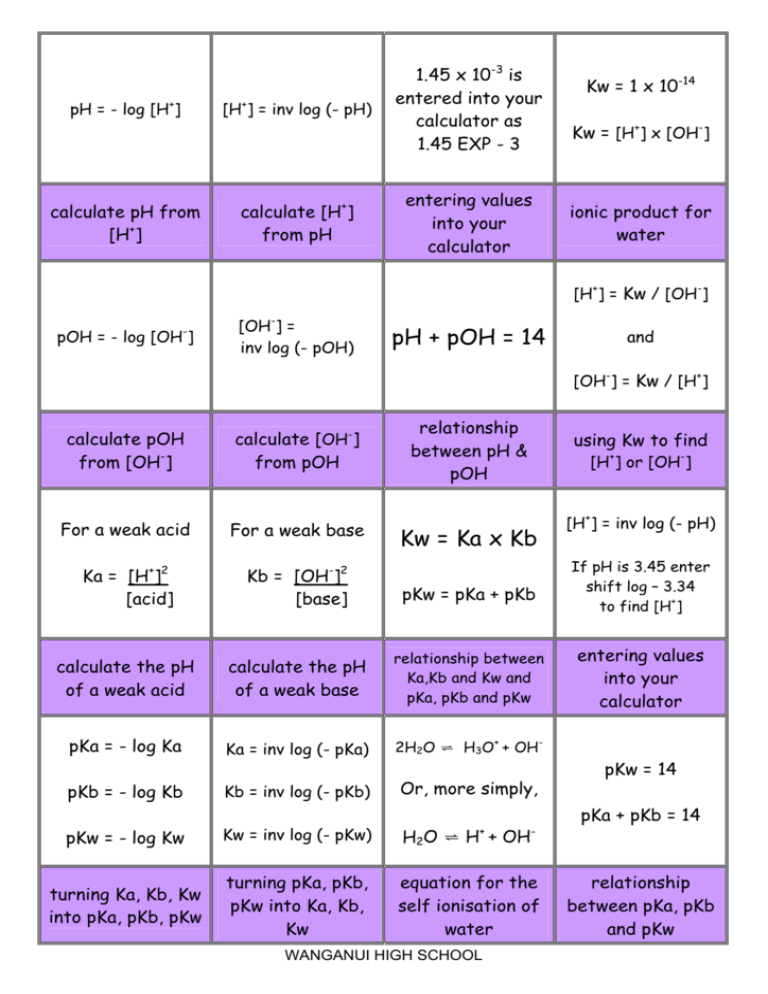
if ionisation constant of an acid HA at equilibrium is 1.0 * 10^ 8, then calculate the value of pKa and pKb (for its conjugate base )

Amazon.com : Calculators Large Display, Durable Financial Calculator Wear-Resistant for Work for Calling for Meeting for Study(Grey, Pisa Leaning Tower Type) : Office Products

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![SOLVED: cc 1. If the Ka of the conjugate acid is 5.14 × 10-7 , what is the pKb for the base? 2. The Kb of dimethylamine [(CH3)2NH] is 5.90x10–4 at 25°C. SOLVED: cc 1. If the Ka of the conjugate acid is 5.14 × 10-7 , what is the pKb for the base? 2. The Kb of dimethylamine [(CH3)2NH] is 5.90x10–4 at 25°C.](https://cdn.numerade.com/ask_previews/65f8ab16-8ad2-4bf2-8167-db27117be525_large.jpg)